COVID-19: FAQs for Sleep Clinicians
The American Academy of Sleep Medicine (AASM) COVID-19 Task Force is responding to frequently asked questions (FAQs) to help sleep clinicians and accredited sleep facilities address the spread of the novel coronavirus (COVID-19). During the COVID-19 public health emergency, the American Academy of Sleep Medicine (AASM) advises sleep clinicians to follow the recommendations of the CDC as well as additional policies, guidance and/or mandates from state and local health departments and your local hospital, health system, institution or jurisdictions. The AASM also offers these considerations for the practice of sleep medicine during COVID-19.
Sleep clinicians and other health care providers can send your sleep-related questions, suggestions and feedback about COVID-19 to the AASM at covid@aasm.org. The AASM also has created a Facebook group to enable sleep professionals to engage with colleagues across the country by discussing clinical challenges such as COVID-19.
Note: Information for patients and the public is available in our blog post, Coronavirus FAQs: CPAP tips for sleep apnea patients. Patients who have questions about their health or medical condition should contact a local medical provider or request a consultation via telemedicine with a provider who is licensed in your state.
Performance of pre-procedure COVID testing may be a consideration for some sleep labs due to reports of breakthrough infections in those who are fully vaccinated. People who get vaccine breakthrough infections can be contagious. The CDC also has noted that the Delta variant is more infectious and is leading to increased transmissibility when compared with other variants, even in some vaccinated individuals.
As of Sept. 10, the infection prevention and control guidance from the CDC is that, “Performance of pre-procedure or pre-admission viral testing is at the discretion of the facility. The yield of this testing for identifying asymptomatic infection is likely low when performed on vaccinated individuals or those in counties with low or moderate transmission. However, these results might continue to be useful in some situations (e.g., when performing higher risk procedures on unvaccinated people) to inform the type of infection control precautions used (e.g., room assignment/cohorting, or PPE used).”
On May 13, the CDC updated its guidance to recommend that fully vaccinated people can resume activities without wearing a mask. However, CDC continues to recommend that patients and visitors should wear a mask upon arrival to and throughout their stay in a health care facility. Similarly, CDC recommendations for use of PPE by health care personnel remain unchanged.
We continue to encourage individuals to follow CDC guidance regarding vaccination. This includes vaccination of health care workers, particularly those who interact directly with patients, and those at risk for severe COVID-19. Newer variants are on the rise, some of which the CDC has indicated as being “of interest” and which the WHO has indicated as being “of concern.” So far, existing vaccines still appear to induce neutralizing antibodies against these variants, even if to a somewhat lower degree.
The risk posed by the newer variants to children also remains under investigation, and children under age 12 are still not approved to receive vaccines. Therefore, we also encourage staff at pediatric sleep centers to follow the vaccination and mask guidance offered by the CDC to mitigate transmission.
According to the “Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination” from the CDC, the recommendations for use of personal protective equipment by health care personnel remain unchanged. In its “Interim Public Health Recommendations for Fully Vaccinated People,” the CDC reports that authorized vaccines in the U.S. are highly effective at protecting vaccinated people against symptomatic and severe COVID-19. Additionally, a growing body of evidence suggests that fully vaccinated people are less likely to have asymptomatic infection and potentially less likely to transmit SARS-CoV-2 to others.
However, how long vaccine protection lasts and how much vaccines protect against emerging SARS-CoV-2 variants are still under investigation. Until more is known, and vaccination coverage increases, some prevention measures will continue to be necessary for all people, regardless of vaccination status. Therefore, at this time, you should continue to follow current mitigation strategies (e.g., universal masking, screening for COVID symptoms, using appropriate PPE) even when staff and patients have been vaccinated.
The decision to conduct testing for COVID-19 prior to potential aerosol-generating procedures should be made on the basis of a risk:benefit assessment by the clinician. Some considerations include: recent COVID-19 infection, local prevalence of newer variants, and vaccination status of others in proximity, to name a few. Refer to the CDC’s “Interim Infection Prevention and Control Recommendations” for a description of the potential benefits and limitations of targeted SARS-CoV-2 testing of patients without signs or symptoms of COVID-19.
Currently, the CDC does not list obstructive sleep apnea as an underlying medical condition that increases the risk of severe illness from the virus that causes COVID-19. However, several of these listed conditions — such as obesity, heart conditions, and Type 2 diabetes — are often shared by individuals who have sleep apnea. The CDC notes that its list is not exhaustive and only includes conditions with sufficient evidence to draw conclusions. Early studies have begun to explore whether existing sleep apnea may increase the risk of COVID-19 infection or COVID-19 mortality and hospitalization; we await more definitive data.
In the interim, it is important to note that healthy sleep has been associated with immunity and vaccination response. Therefore, we encourage practices that promote healthy sleep, including treatment for existing sleep disorders.
Regarding the duration of isolation and precautions for adults with COVID-19, the CDC recommends a symptom-based strategy, rather than a test-based strategy, for ending isolation of these patients. The CDC reports that people with mild to moderate COVID-19 remain infectious no longer than 10 days after symptom onset. People with more severe to critical illness or severe immunocompromise likely remain infectious no longer than 20 days after symptom onset. Although rare, CDC cautions that some people who have COVID-19 may become susceptible to reinfection, beginning around 90 days after symptom onset. Therefore, when scheduling care (e.g., sleep study) for a COVID-positive patient, consider scheduling the patient:
- at least 10 days after symptom onset (or after a positive test if the patient never developed symptoms); OR
- at least 20 days after symptom onset if the illness was severe; OR
- if at least 90 days have elapsed since symptom onset, consider pre-appointment screening.
Some laboratories may wish to implement a simplified protocol to triage patients with a known history of recent COVID-19 infection. If such a patient requires in-laboratory testing, consider waiting a minimum of 20 days until after symptom onset before scheduling. If an earlier appointment is deemed clinically necessary, consider having a sleep medicine professional review the patient’s presentation and conduct a risk/benefit analysis to justify earlier testing.
Sleep facility leaders should encourage staff and patients to be vaccinated in accordance with CDC guidance. Hospitals and health systems that are prioritizing clinical staff for vaccination should include sleep technologists (both part-time and full-time) among those who are prioritized to receive the vaccine. Sleep clinicians should address vaccine status at appointments, be prepared to answer questions and offer education to patients, and encourage healthy sleep habits before and after vaccination, which may help optimize the immune response.
While COVID-19 vaccine approval by the FDA is an important development, you should anticipate that vaccine distribution will take time. Variations in states and localities may exist in distribution protocols and schedules. Even after vaccination, we encourage continuation of mitigation strategies. The CDC emphasizes that it is important for everyone to continue following the CDC recommendations for how to protect yourself and others. Vaccine resources available from the CDC include clinical resources, recipient education, and a communication toolkit for medical centers and clinicians.
Among patients, post-COVID complaints of changes in sleep quality and quantity have been reported, including complaints of unrefreshing sleep, insomnia, and fatigue. Additionally, those with long-term lung injury may be at greater risk for developing sleep related breathing disorders. An evaluation by a sleep clinician is encouraged to rule out treatable disorders, including obstructive sleep apnea, and sleep related hypoxia.
Although no COVID-specific interventions can be recommended at this time, the AASM does recommend that clinicians use multi-component cognitive behavioral therapy for insomnia for the treatment of chronic insomnia disorder in adults. While sleep hygiene may be helpful for transitory insomnia symptoms, it should not be used as a single-component therapy for chronic insomnia. A sleep diary may be useful during evaluation and help identify phase disorders, sleep hygiene practices, and other commonly encountered conditions.
Some data suggest that individuals with OSA may be at higher risk for severe COVID-19 infection, if they were to acquire it. Therefore, we encourage patients with OSA to adhere to CDC guidelines regarding reducing the risk of transmission, including wearing a face covering, maintaining physical distancing, avoiding crowded places, and staying home if sick.
- Consider advising patients to bring in their own device and mask interface; limit staff going into rooms to do mask fittings or prescription adjustments.
- Request that hospitals designate an appropriate individual to help with decision-making, balancing the needs of patients and public safety.
During the public health emergency related to the ongoing spread of COVID-19, decisions to restrict, cease, or resume sleep-related health care services should be guided by state executive orders, local transmission levels, and directives from state and local departments of health.
Sleep clinicians should be prepared to adjust operations as local conditions change, with the expectation that intermittent, short-term restrictions or closures may be needed in response to sudden surges in local community transmission.
Here are the general definitions provided by the CDC for the local level of community transmission of COVID-19:
- Substantial community transmission: Large-scale community transmission, including within communal settings (e.g., schools, workplaces)
- Minimal to moderate community transmission: Sustained transmission with high likelihood or confirmed exposure within communal settings and potential for rapid increase in cases
- No to minimal community transmission: Evidence of isolated cases or limited community transmission, case investigations underway; no evidence of exposure in large communal setting
Trends in local transmission should be viewed over time using available county-level data. Sources for these data include the CDC and others, such as USA Facts, Johns Hopkins University, the University of Washington, and Harvard Global Health Institute. Local information may be more relevant, and we encourage practices to obtain data regarding prevalence, which may be inferred from rates of testing and from rates of patients with positive tests.
When testing is inadequate or insufficient, surrogate markers that are more robust (but may take longer to identify local prevalence and trends) include: hospital admissions, ventilator use, and mortality rates due to COVID-19. These rates, however, depend on the subgroups affected; for example, communities with widespread prevalence among younger and healthier groups may not see an increase in hospitalizations or deaths unlike communities in which older adults with risk factors for severe disease are infected. Judgement and interpretation are required, as current metrics may not be up-to-date in every locality. Local health departments and hospitals may provide more information.
The CDC states that, “Routine cleaning and disinfection procedures (e.g., using cleaners and water to pre-clean surfaces prior to applying an EPA-registered, hospital-grade disinfectant to frequently touched surfaces or objects for appropriate contact times as indicated on the product’s label) are appropriate for SARS-CoV-2 in healthcare settings, including those patient-care areas in which aerosol generating procedures are performed.
“Refer to List N on the EPA website for EPA-registered disinfectants that have qualified under EPA’s emerging viral pathogens program for use against SARS-CoV-2. Management of laundry, food service utensils, and medical waste should also be performed in accordance with routine procedures.”
The CDC has standard guidelines for cleaning carpeting and cloth furnishings as well as laundry and bedding.
The CDC states that, “AIIRs are single-patient rooms at negative pressure relative to the surrounding areas, and with a minimum of 6 air changes per hour (12 air changes per hour are recommended for new construction or renovation). Air from these rooms should be exhausted directly to the outside or be filtered through a high-efficiency particulate air (HEPA) filter directly before recirculation. Room doors should be kept closed except when entering or leaving the room, and entry and exit should be minimized. Facilities should monitor and document the proper negative-pressure function of these rooms.”
The CDC has more information about airborne infection isolation.
The COVID-19 section of the CDC website has information and recommendations for health care providers, including:
- Resources for Healthcare Facilities
- Information for Healthcare Professionals
- Transmission-Based Precautions
- Mitigation Recommendations
- Guidance for Evaluating Persons Under Investigation (PUI)
- Healthcare Professionals: Frequently Asked Questions and Answers
- COVID-19: The Internet Book of Critical Care
The CDC Clinician Outreach and Communication Activity (COCA) hosted a live webinar on Friday, March 13, “Coronavirus Disease 2019 (COVID-19) Update and Infection Prevention and Control Recommendations.” (Updated March 14)
- Stay up to date on CDC, state, and local public health recommendations based on community risk, as this is an evolving situation.
- Screen patients and visitors (whose access to clinical areas should be restricted, as per CDC recommendations) for risk before entering your health care facility. To do this, use guidance criteria offered by the CDC, state and local government, and county or other health departments. Follow your institution’s recommended protocols regarding triage.
- If telemedicine visits are available, offer this option to your patients in a pre-emptive fashion.
- Personnel who develop respiratory symptoms (e.g., fever, cough, shortness of breath) should be instructed not to report to work. Ensure that your sick leave policies are flexible and consistent with public health guidance and that employees are aware of these policies.
- Ensure clear lines of communication on policy changes with staff.
- Encourage adequate rest and recovery periods for your health care workforce.
The CDC currently does not have guidance specific to sleep centers and laboratories. In the interim, members are invited to consider the following:
UPDATE: The AASM has posted “Summary of CDC recommendations relevant for sleep practices during COVID-19” and “Considerations for the practice of sleep medicine during COVID-19.” (Aug. 27)
Develop clear and specific algorithms for patient flow
- Follow institutional and CDC guidelines.
- Consider use of available advice lines, patient portals, and online self-assessment tools for risk assessment.
- Consider postponing appointments, or use of telephone or virtual visits, whenever possible.
- Consider issues regarding safety-sensitive work among your patients.
- Follow institutional protocols and CDC guidelines regarding risk assessment on an ongoing basis after arrival at the facility.
Administration of positive airway pressure (PAP) therapy in known or suspected COVID-19 patients
- Published data is limited and suggests that there may be the potential to increase particle dispersion during the use of positive airway pressure therapy.
- During the outbreak of SARS-CoV in Toronto, half of all SARS-CoV cases were in health care workers, three of whom died, despite existing safety protocols. The greatest risk of becoming infected was experienced by those involved in manipulating the airway or those who were exposed to aerosolized pathogens via nebulizers, positive airway pressure therapy, or high flow nasal oxygen therapy.
- Therefore, in general, when the possibility of increased risk of infecting others exists, positive airway pressure therapy delivered via mask should be based on a thorough risk-benefit analysis if a patient has COVID-19.
Cleaning of equipment
- Individual facilities are urged to follow CDC recommendations regarding cleaning of facilities and equipment.
UPDATE: The AASM has posted “Summary of CDC recommendations relevant for sleep practices during COVID-19” and “Considerations for the practice of sleep medicine during COVID-19.” (Aug. 27)
The decision to limit or halt sleep services should be based on CDC mitigation strategies as well as guidance from local and regional health authorities. If the lab is within a health system, institutional policies should also be consulted.
In some states with anticipated acceleration in community-based spread, some independent sleep laboratories are canceling non-essential diagnostic testing until otherwise advised by their health system and local, state and CDC officials.
For the short-term, patient education should be offered regarding risk-mitigation strategies to avoid adverse consequences of untreated sleep apnea. This includes, in particular, the acute risk of fall-asleep crashes. Patients should be advised to: pursue non-PAP therapies, including positional therapy; limit the use of alcohol and sedating medications; control nasal congestion; and continue efforts at weight management.
UPDATE: At this time, the airborne route is presumed to be the major route of transmission of COVID-19. As such, many laboratories no longer quarantine HSAT devices upon receipt. One should continue to follow manufacturer instructions regarding cleaning and disinfection between uses. (Updated March 26, 2021)
Some sleep laboratories are dispensing HSAT units through mail delivery to reduce patient contact, while others are suspending services for a limited time, with decisions made on a case-by-case basis. Exceptions may be required when there is an intolerable risk of acute adverse outcomes, such as an unavoidable emergency or safety-sensitive activity (e.g., emergency workers who report drowsiness while driving).
If an HSAT is performed, some labs are waiting for at least 3 days before handling the equipment. Thorough manufacturers’ cleaning instructions should be followed. Universal precautions should be followed by personnel handling recovered testing equipment.
We recommend following manufacturer recommendations* and using existing CDC guidelines regarding cleaning and disinfection. Upon a patient’s recovery from COVID-19, it may be advisable to replace filters, given the lack of data regarding the possibility of re-infection.
*Keeping it clean: CPAP hygiene (Philips)
*How to clean your CPAP equipment (ResMed)
If a patient is suspected or confirmed to have COVID-19, we suggest assessing risks and benefits of continuing to use a PAP (CPAP/BPAP) device at home.
Considerations include:
What are the risks of continuing PAP therapy?
- There may be increased risk of transmission of COVID-19 to others in the environment if PAP is continued.
- Consider individuals residing in proximity to the patient, especially if they are at risk for severe infection. Dispersion of the virus with PAP is theoretically greater with than without PAP, but how much the risk to others changes specifically because of PAP therapy is not known.
- Viral particles may persist for some time depending on the type of surface.
- Persons at risk for infection from using PAP include co-habitants of the same dwelling.
- Additionally, whether it is possible for the patient to be re-infected from tubing, filters, and/or mask reuse is not known.
What are the risks of discontinuing PAP therapy?
- OSA is a chronic disorder, and the risk of stopping PAP for a limited period of time until the patient is no longer contagious may be manageable, depending on the severity of the disorder and symptoms. Without PAP, however, some patients may experience an increase in health risks in the short term, such as accidents, safety incidents, falls, or cardiovascular events.
- If such acute risks are identified, risk-mitigation strategies may be appropriate, such as advising the patient to stop driving, adhere to fall precautions, and consult with their treating physician to optimize medical management of background medical conditions.
- Using positional therapy or an oral appliance (if the patient already has one), limiting the use of alcohol and sedating medications, and addressing nasal congestion may also be effective for some patients.
- If these short-term risk mitigation strategies are insufficient, and a decision is made to continue PAP in a patient who has confirmed COVID-19, or is suspected of having COVID-19, the patient should be advised to maintain strict quarantine and consider strategies for protecting household contacts.
The decision of whether to continue or stop PAP therapy should be based on whether the risk:benefit assessment favors continued therapy.
There is regional and payor variability in response to this situation. While some private insurers are moving quickly to offer reimbursements for services without in-person visits, others have not yet done so. The AASM sent a letter to CMS requesting a waiver of the in-person PAP requirements and is awaiting a response. In the meantime, CMS announced that it is temporarily expanding telemedicine coverage in response to COVID-19. CMS indicates that for the duration of the COVID-19 public health emergency, Medicare will make payment for telemedicine services furnished to patients in all areas of the country and in all settings, including the home. These visits are considered the same as in-person visits and are paid under the Physician Fee Schedule at the same rate as regular, in-person visits. The expanded coverage also includes virtual check-ins and e-visits. During this national emergency, telemedicine visits should be pursued whenever allowable.
We also urge sleep physicians to take an active role in advocating for local policies that address the needs of their patients and staff regarding response to COVID-19, since they are positioned to assess local risks. We urge everyone to stay abreast of recommendations from CDC and local health departments.
**UPDATE – CMS announced on March 30 that it has released an interim final rule summarizing revisions to CMS processes allowing for increased flexibility in providing safe and effective care during the COVID-19 pandemic. The sweeping, temporary changes were made to promote the widespread use of telecommunications technology and avoid exposure risks to health care providers, patients, and the community during this outbreak. These regulations are applicable beginning March 1, 2020, lasting throughout the national public health emergency as declared by the secretary of Health and Human Services (HHS).
Let’s break this up into two parts: 1) Is PAP aerosol-generating? 2) Can aerosols spread virus?
Question 1: Currently, this is the thinking:
“NIV is a potential aerosol-generating device. In this regard, the deliberate leakage via the exhalation ports may generate droplet nuclei and disperse infective aerosols through the evaporation of the water content of respiratory droplets resulting in a superspreading event.”
Source: Singh A, Singh J. Noninvasive ventilation in acute respiratory failure due to H1N1 influenza: A word of caution. Lung India. 2011 Apr;28(2):151. https://doi.org/10.4103/0970-2113.80340
Also see: Tang JW, Li Y, Eames I, Chan PK, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64:100–14. https://doi.org/10.1016/j.jhin.2006.05.022
“Until further data become available, it should be assumed that [noninvasive ventilation] is aerosol generating…Transmission of COVID-19 is primarily through droplet spread. These droplets are affected by gravity and may cause direct transmission from close contact or contribute to surface contamination (where the virus may remain active for hours to days). However, positive pressure ventilation is thought to generate aerosols composed of smaller virus containing particles suspended in air. These airborne particles may travel greater distances and be inhaled, increasing the risk of transmission.”
Source: Brewster DJ, Chrimes NC, Do TBT, et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. The Medical Journal of Australia. Preprint only. Updated March 17, 2020. Accessed March 24, 2020.
Question 2: Virus has been shown to remain viable for hours after aerosolization:
“aerosol and fomite transmission of the virus is plausible, since the virus can remain viable and infectious in aerosols for hours and on surfaces up to days (depending on the inoculum shed).”
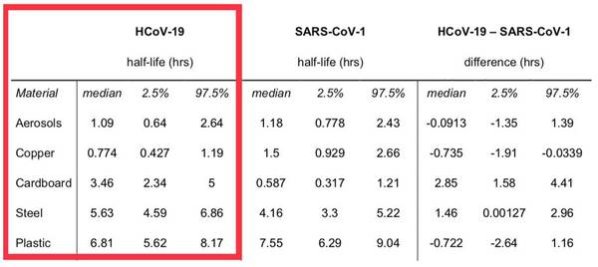
Source: van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Mar 17. https://doi.org/10.1056/NEJMc2004973
(Image: Supplementary Appendix)
It’s best for your patients to contact their DME provider to get the latest recommendations. These recommendations suggest that optimal humidifier performance requires distilled water. If distilled water is not available, alternatives for short-term use are purified bottled water (without added minerals) or tap water (preferably filtered). If these options are chosen, more rigorous humidifier cleaning is advised to prevent excess mineral buildup in the tub. Water that might not be treated or purified (well water, for example) should be avoided. Here is a link to make distilled water at home.
ResMed also provides this advice: “As stated in our clinical and user guides, *optimal* humidifier performance requires distilled water. That’s because most or all of its minerals have been removed, preventing mineral buildup in the humidifier tub. That said, tap or bottled water may also be used. It will not harm the device or pose a risk to patients. It will, however, require more rigorous humidifier cleaning to prevent excess mineral buildup in the tub.”
When possible, the AASM recommends referring to guidance issued by local health centers or hospitals, due to regional variation in prevalence and resources. At a minimum, AASM suggests following CDC recommendations regarding aerosolizing procedures.
If COVID-19 status is unknown or positive, recognize that PAP/NIV may increase aerosolization, and therefore, potential transmission of virus.
Therefore, NIV/PAP should be avoided as much as possible.
Among individuals in whom COVID-19 testing is negative, any decision to use PAP/NIV should take into careful consideration the following:
- Estimated background prevalence rates; under conditions of high background prevalence, the likelihood of false negative (unrecognized cases) is expected to be higher. Therefore, even patients whose COVID-19 testing is negative should be treated with caution. Note that reported rates of false negative rates have varied.
- Whether PAP is absolutely necessary, based on assessment of acute/short-term health or safety risks of temporary withdrawal of therapy. In typical settings, we expect such need to be infrequent.
- The ability to properly protect health care professionals and other patients, through access to properly-ventilated rooms, PPE, and other strategies to limit viral dispersion to others.
Alternatively, for short-term treatment of OSA while hospitalized, we highly recommend conservative measures such as elevating the head of the bed when oxygenation status permits, or using prone positioning. If appropriate, consider the use of low-flow oxygen via nasal cannula. Limit airway manipulation or procedures that may increase risk of spread of the virus.
Updated March 26, 2021




