Compumedics announced March 5 that its Falcon HST device received FDA 510(k) clearance, expanding the company’s home sleep testing lineup in the U.S.
About Falcon HST
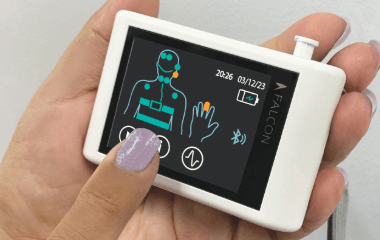
Falcon HST is an electroencephalogram (EEG) and respiratory signal recorder. It records, reviews and analyzes collected and stored physiological parameters, including EEG, electrooculogram (EOG), electrocardiogram (ECG) and respiratory signals, which are used as an aid in the diagnosis of respiratory- and cardiac-related sleep disorders by qualified physicians.
The Falcon system consists of a main unit and a charging cradle. The main unit is a small device worn on the chest over clothing. It includes a touchscreen LCD and various channel inputs for plethysmography bands and electrodes.
The device is available by prescription only, and is intended for use by adult patients in the home or clinical environment, under the direction of a health professional, to aid in the diagnosis of sleep disorders.
According to the company’s press release, the device complements Somfit, providing a clinically focused platform with more data points. Somfit is a wearable device intended for home use with patients suspected of having sleep-related breathing disorders. Worn on the forehead, the device collects physiological data and is intended to be a diagnostic aid for clinicians. Somfit received FDA clearance in December 2023.
Read more industry news from the AASM.