On March 20, Sibel Health announced its ANNE View and Central Hub received FDA 510(k) clearance to display data from ANNE devices and third-party monitors, providing alerts, patient positioning insights and multi-patient monitoring to support health care professionals.
About ANNE View, Central Hub
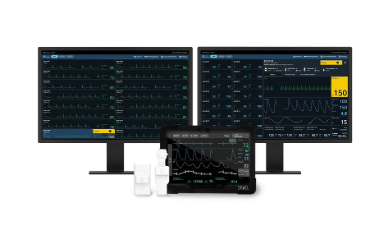
The ANNE View application, paired with the Central Hub, offers a bedside patient monitoring system designed to support health care professionals in clinical and home care environments.
ANNE View collects and displays vital signs and physiological data from the ANNE Chest and ANNE Limb devices. When connected to ANNE Chest, the ANNE View application displays electrocardiography (ECG), skin temperature, heart rate, respiratory rate and body position data. When connected to ANNE Limb, ANNE View displays photoplethysmography (PPG), SpO2, pulse rate and skin temperature data.
It is also compatible with third-party, FDA-cleared devices for the display of noninvasive blood pressure, SpO2, pulse rate and body temperature measurements.
A feature of ANNE View is its alert system, which notifies health care staff when a patient’s data falls outside pre-set parameters. Additionally, the system monitors patient positioning, helping prevent pressure ulcers by alerting caregivers if a patient remains in the same position for too long. This aids in timely intervention for at-risk individuals.
ANNE View communicates with compatible central monitoring platforms, including the Central Hub, for the display and storage of multiple patients’ physiological data. It allows health care professionals to view data from up to 16 beds on one screen and stores up to 48 hours of patient data. Visual and audio alerts are relayed from ANNE View to the Central Hub, keeping health care teams informed of changes in a patient’s condition.
Sibel Health received FDA 510(k) clearance for its ANNE One platform in May 2023.
Read more industry news from the AASM.