In March 2024, the AASM hosted a webinar featuring an interactive panel discussion addressing uncertainties stemming from the announcement by Philips Respironics that it is discontinuing various sleep and respiratory care devices in the U.S. The discussion also addressed the consent decree between Philips, the U.S. Food and Drug Administration (FDA), and U.S. Department of Justice (DOJ) related to the ongoing recall of Philips positive airway pressure (PAP) devices.
Representatives from the AASM, American College of Chest Physicians (CHEST), American Thoracic Society (ATS), American Academy of Neurology (AAN), American Association for Respiratory Care (AARC), and American Association of Sleep Technologists (AAST) discussed the implications for sleep centers. A video recording of the webinar is available for viewing on the AASM YouTube page.
The webinar generated multiple questions from participants, and the AASM Public Safety Committee has provided the following responses to address common inquiries. Sleep medicine professionals should reach out to your Philips Respironics sales representative or Customer Service at 800-345-6443 with additional questions.
Questions and Answers
How does the consent decree impact patients and labs?
The consent decree is a legally binding performance improvement plan. It has outlined a roadmap of what actions and milestones Philips Respironics must meet to demonstrate regulatory compliance and restore its business in respiratory and sleep services in the U.S. The structure outlined in the decree includes prioritizing remediation of sleep and respiratory devices recalled in June 2021, establishing independent experts involved in the recall remediation, and ensuring independent review of compliance. No further sale of BiPAP or CPAP devices within the U.S. is allowed until Philips meets these requirements. For further information, read the AASM summary of the final agreement.
What is involved in the Philips announcement of sleep and respiratory portfolio changes?
Based on the announcement, for the U.S. and U.S. territories, Philips Respironics will shift its focus to the sale of consumables and PAP accessories, including masks, and will not return to the sale of hospital ventilation products, certain home ventilation products, portable and stationary oxygen concentrators, and sleep diagnostic products. In addition, a portion of non-U.S. sales will compensate the FDA for fines levied by the recall. More details are available in the Philips portfolio update. See the table below for a list of discontinued products.
What defines the “end of service” for Philips products in the portfolio change?
After Philips discontinues a product for sale and discontinues shipments, there will be a specified number of years of service to support all relevant repair parts and accessories necessary to use or service the device pending part availability. Beyond the end of service date for a selected device, Philips will no longer support or provide service. Be aware that the service, replacement parts and accessories are subject to availability for listed devices. In other words, if they are unavailable, access to parts and accessories may not be provided. See the table below for specific end of service dates.
Many of our Philips titration devices (i.e., OmniLab Advanced+) continue to be used since there is limited availability of an alternative. How do I cover the cost? Is there any support?
In January 2024, Philips Respironics discontinued sales and shipment of the OmniLab Advanced+ in the U.S. Based on the consent decree, Philips can continue to service existing devices and provide accessories and replacement parts based on availability. Philips indicates that the end-of-service date for these devices is Jan. 25, 2029. See the table below.
What are the options if a discontinued Philips product has failed within its warranty period and is non-repairable?
Philips will provide refunds as articulated in the warranty terms and conditions but will be unable to provide a replacement product.
Many of my patients have limited resources and clinically benefit from their CPAP. They are afraid about the ongoing use of a Philips product and cannot afford an alternative. Are there any resources or solutions for these patients to receive an alternative treatment?
Depending on the device being used, your patients’ options following registration of their device may include financial payment. This may support the cost of new treatment, but full treatment replacement or support of an alternative is not part of the consent decree. More information is available from Philips on the remediation pathway webpage. It is important to note this registration deadline: Registration for affected CPAP and BiPAP devices in the U.S. and Canada ends at 11:59 p.m. EST, Dec. 31, 2024. To receive a replacement device or financial payment, the device must be registered with Philips prior to the end of the year.
What are some considerations for patients who have limited resources and are dependent on a Philips Respironics device?
Some patients are financially unable to obtain new equipment (including maintenance products) or transition to alternative therapy. Further, payers may not have changed or accommodated their health care policies to allow a transition to another PAP device or respiratory assist device (RAD). Although remediation will be provided by Philips, a full transition may be incomplete in some cases. In addition, there are situations in which DME companies are no longer servicing Philips devices. This has created challenges that may allow for unforeseen difficulties for these patients.
Due to the menu of devices that have been impacted, and patients’ dependence on Philips Respironics devices (in some cases for years), sleep and respiratory professionals should consider patients’ comfort level and challenges in transitioning (e.g., device difference, retesting), recognize vulnerable population perspectives (e.g., loss of trust), and specific needs (e.g., education, language) as well as intensify collaboration with local and regional DME suppliers.
What issues are being encountered by sleep labs, sleep and respiratory practices, and DME suppliers who are trying to coordinate care?
Due to the size and scope of the Philips recall, and the current resource limitations across the broad array of products, it is anticipated that this announcement is broadly felt by:
- Sleep labs: Transitioning from a Philips titration device to another involves other costs including accessories (e.g., interface cables), technician training, and other unmeasured expenses.
- Sleep and respiratory practices: Due to insurance requirements, practices are realigning patient products, coordination of care, and alternative approaches to medical equipment.
- DME suppliers: To provide access to devices, DME suppliers sought alternatives from nontraditional, foreign, and domestic manufactures with unknown or unclear (or not fully vetted) quality assurance programs.
What are some of the challenges from having too few manufacturers making equipment?
Since the recall, new treatments and manufacturers have entered the marketplace, partially filling a gap in a brief period. Since these new therapeutics did not have time to mature, and supply chains were limited, there were mounting concerns about quality challenges. However, greater competition is going to be beneficial for patients in the future.
What does the Philips announcement mean for the device marketplace; is it adequate to serve patients? What is the outlook for the future in sleep and respiratory care?
Since the initial Philips recall announcement in 2021, and their portfolio changes in 2024, there has been a growth of traditional and alternative manufacturers in the sleep and respiratory market. Previously, there was limited product availability followed by an intermediate recovery. It is anticipated that with an enhanced regulatory environment, longitudinal expectations support an improved supply of sleep and respiratory care devices and better care for patients. A call for better care has led to the pursuit of more head-to-head trials comparing therapeutics and devices, and there is a greater understanding of the gaps in care for our most vulnerable patients who rely on sleep and respiratory devices.
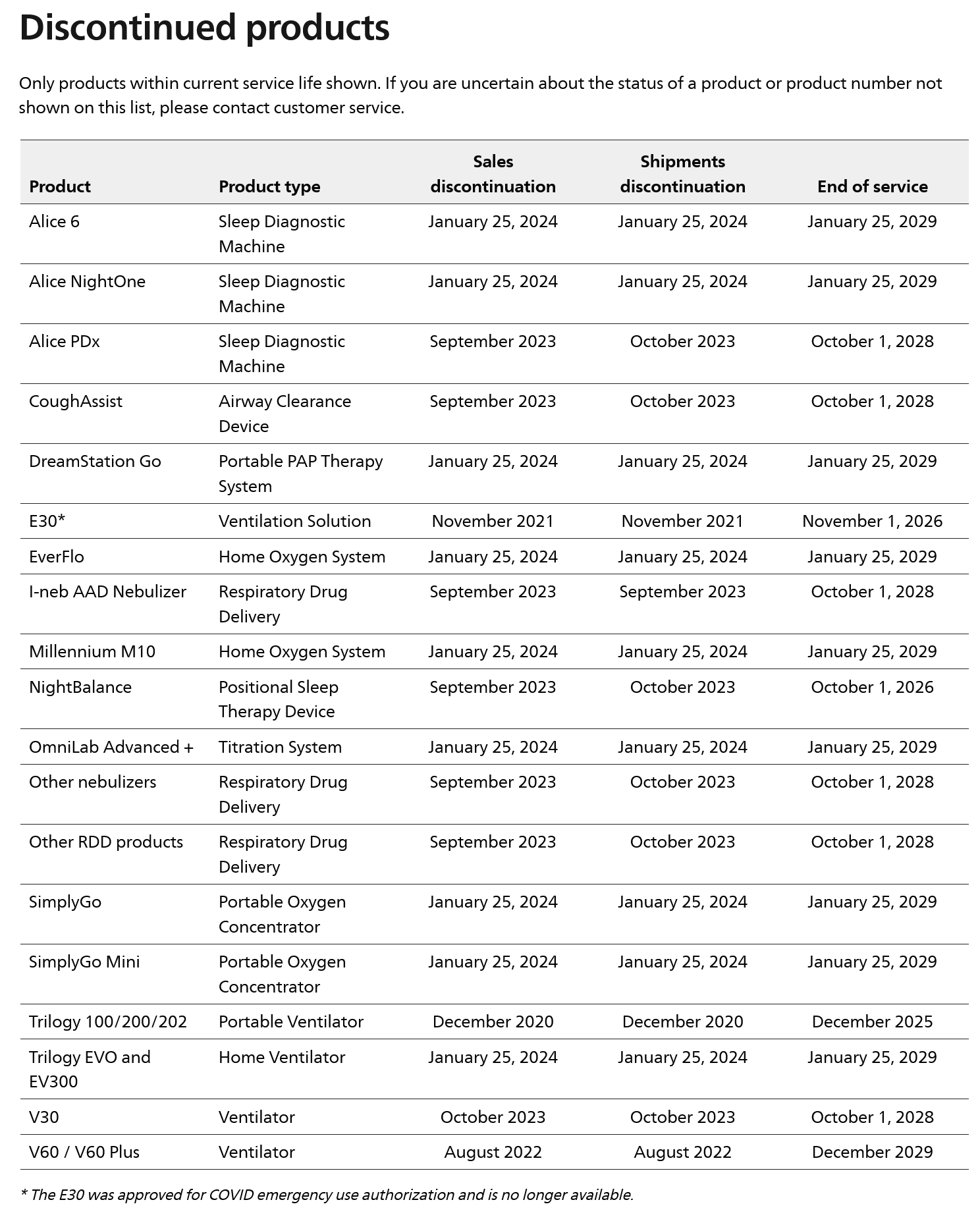
Source: Philips Respironics. Sleep & Respiratory Product Portfolio Changes.




