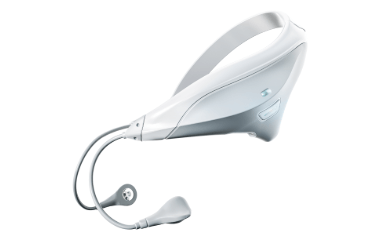
On Oct. 28, 2024, Neurovalens announced it launched its first product, Modius Sleep, an FDA-cleared, physician-prescribed medical device intended to treat chronic insomnia in adults.
About Modius Sleep
Modius Sleep is a portable, battery-operated neurostimulation device worn on the head for 30 minutes each evening. It sends a low-level electrical pulse into the vestibular nerve, influencing areas of the brain responsible for controlling circadian rhythm and sleep patterns. The neurostimulation is delivered through two self-adhesive electrode pads placed on the skin behind the ears. The intensity of the electrical pulse can be adjusted by the user. It is intended as a non-invasive therapeutic method to improve chronic insomnia in adults aged 22 years and older.
Results of a clinical trial published in the journal Brain Stimulation suggest that, after a four-week intervention, Modius Sleep significantly improved insomnia severity compared with a sham device, with the most benefit seen in those with severe insomnia.
Modius Sleep received FDA clearance in Oct. 2023 and is now available in the U.S. by prescription.
About chronic insomnia
Chronic insomnia is a sleep disorder characterized by persistent difficulties in falling or staying asleep, resulting in disrupted sleep patterns and daytime symptoms. It significantly impacts various aspects of life, affecting work performance, decision-making, relationships, mood and overall quality of life. Approximately 10% of individuals experience chronic insomnia, defined by sleep disturbances occurring at least three times a week and lasting for a minimum of three months.
Read more industry news from the AASM.