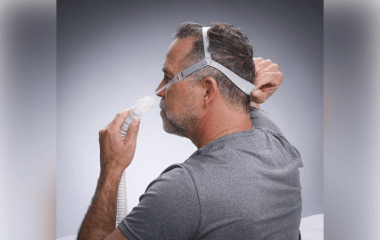
In July 2024, REMSleep Holdings received FDA 510(k) clearance for its DeltaWave Nasal Pillow System, a CPAP interface mask designed to improve comfort and airflow for patients undergoing positive airway pressure therapy.
About REMSleep’s DeltaWave mask
The DeltaWave Nasal Pillow System is a noninvasive device that channels pressurized airflow from a CPAP or bilevel machine to the patient’s nasal passages. It features nasal pillows that gently dilate the nostrils, helping to reduce airflow restrictions and allowing air to enter at a lower driving pressure. This design aims to maintain consistent pressure and improve the efficiency of therapy.
The system includes single-strap headgear, flexible tubing and a swivel adapter, and it connects to CPAP devices using a standard conical connector. According to the company, the mask is engineered to support natural breathing by applying principles of fluid dynamics, helping users inhale and exhale with minimal resistance. This may allow patients to breathe more comfortably at their normal respiratory rate without mechanical disruption.
Additional benefits highlighted by REMSleep include low operational noise, a simple and loose-fitting head strap, and potentially reduced need for humidification. The DeltaWave is intended for use in adults weighing at least 66 pounds who have been prescribed CPAP or bilevel therapy.
About obstructive sleep apnea
Obstructive sleep apnea is a serious sleep disorder where the airway becomes repeatedly blocked during sleep, causing pauses in breathing. This condition, often marked by loud snoring and choking noises, can lead to oxygen deprivation, disrupting sleep and contributing to various health issues such as high blood pressure, heart disease, stroke and diabetes.
Read more industry news from the AASM.