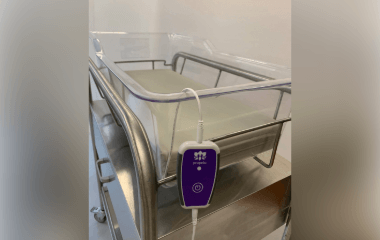
On April 4, the FDA granted De Novo clearance to the Prapela SVS hospital bassinet pad, a vibrating mattress pad designed to stimulate rhythmic breathing in premature newborns with neonatal opioid withdrawal syndrome (NOWS) through gentle, randomized movements.
About Prapela SVS hospital bassinet pad
The Prapela SVS hospital bassinet pad is a prescription-only therapeutic medical device that delivers stochastic vibrotactile stimulation (SVS), a form of gentle, randomized vibration. It is intended as an adjunctive, non-pharmacological therapy for newborns with a gestational age of 37 weeks or less who were prenatally exposed to opioids and diagnosed with NOWS.
Infants with NOWS often experience dysregulation of the nervous system, disrupted sleep, and abnormal movement and muscle tone. The Prapela SVS pad is designed to provide low-level stimulation that resonates with nerve endings to help support the brain’s control over these functions. The gentle, randomized movement is not strong enough to wake sleeping infants but is intended to encourage more stable breathing rhythms.
The pad can be used within 48 hours of birth and is compatible with standard hospital bassinets, radiant warmers and incubators. It features a firm, flat surface that aligns with the American Academy of Pediatrics safe sleep recommendations. The vibration is activated by a single button and the device is reusable.
In a randomized clinical trial of newborns with prenatal opioid exposure published in JAMA Pediatrics, infants who received SVS had fewer days in treatment and required lower doses of morphine to manage withdrawal symptoms.
The device is intended for use in professional health care settings with temperature-controlled environments, such as neonatal step-down units, hospital patient rooms and offsite specialty centers.
Read more industry news from the AASM.