The U.S. Food and Drug Administration today announced the approval of a new treatment option for patients who have been diagnosed with moderate to severe central sleep apnea. The Remedē System is an implantable device that stimulates a nerve located in the chest that is responsible for sending signals to the diaphragm to stimulate breathing. The FDA granted approval of Remedē System to Respicardia Inc.
The Remedē System comprises a battery pack surgically placed under the skin in the upper chest area and small, thin wires that are inserted into the blood vessels in the chest near the nerve (phrenic) that stimulates breathing. The system monitors the patient’s respiratory signals during sleep and stimulates the nerve to move the diaphragm and restore normal breathing.
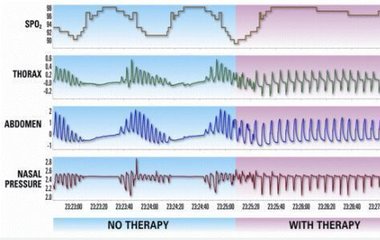
The FDA evaluated data from 141 patients to assess the effectiveness of the Remedē System in reducing apnea hypopnea index (AHI), a measure of the frequency and severity of apnea episodes. After six months, AHI was reduced by 50 percent or more in 51 percent of patients with an active Remedē System implanted. AHI was reduced by 11 percent in patients without an active Remedē System implanted.
This device is not intended for use in patients with obstructive sleep apnea, a condition in which the patient attempts to breathe, but the upper airway is partially or completely blocked. In 2014 the FDA approved the upper airway stimulation therapy by Inspire Medical Systems for use in a subset of patients with moderate to severe obstructive sleep apnea who are unable to use continuous positive airway pressure (CPAP) therapy.
UPDATE: Respicardia announced on Oct. 20, 2020, 5-year results from the remedē System Post Approval Study, assessing the safety and efficacy of transvenous phrenic nerve stimulation for the treatment of moderate to severe central sleep apnea. Highlights from the 5-year data show sustained improvements from baseline that were highly consistent with the previously published 6-, 12-, 24-, and 36-month results.
Read more industry news from the AASM.
Image from Respicardia Inc.
Updated Oct. 21, 2020